Multiple Choice
Given the accompanying phase diagram, under what conditions will liquid be found in equilibrium with either solid or gas? 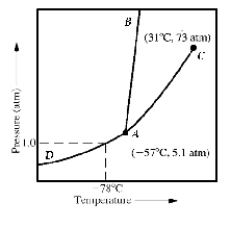
A) Anywhere along curve AB
B) Anywhere along curve AC
C) Anywhere along curve AD
D) Anywhere along curve AB and AC
E) Anywhere along curve AB and AD
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Strontium oxide has a face-centered cubic unit
Q5: The metal vanadium crystallizes in a body-centered
Q7: If an ionic solid has a face-centered
Q15: Using the thermodynamic data below and
Q25: According to the below phase diagram, what
Q28: Chromium (atomic mass 52.00 g/mol)crystallizes in a
Q41: Above a substance's _ temperature,it is not
Q43: Calcium sulfide has a face-centered cubic unit
Q48: Which of the following elements might be
Q50: Nickel has a face-centered cubic cell,and its