Multiple Choice
Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C−H σ bonding molecular orbital in ethene, H2C=CH2 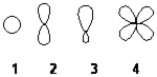
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Consider the structure of urea given below.
Q35: What is the molecular formula of Ritalin,
Q36: What is the ground-state electronic configuration of
Q37: Which of the following molecules has a
Q38: Which of the following molecules has a
Q40: Which of the 3 structures shown below
Q41: Draw bond-line structures of all of the
Q42: The following molecules all contain the same
Q43: What is the approximate value of the
Q44: Consider the following molecular model. <img