True/False
Consider the structure of urea given below. 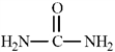 To complete the Lewis structure, six nonbonding electrons should be added, two to each of the nitrogen atoms and two to the oxygen atom.
To complete the Lewis structure, six nonbonding electrons should be added, two to each of the nitrogen atoms and two to the oxygen atom.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Which of the following species possesses a
Q30: Which of the following is a tertiary
Q31: Which of the following statements is not
Q32: Fill the appropriate electronic configuration in the
Q33: Which of the following bonds is the
Q35: What is the molecular formula of Ritalin,
Q36: What is the ground-state electronic configuration of
Q37: Which of the following molecules has a
Q38: Which of the following molecules has a
Q39: Which of the following best represents an