Multiple Choice
Propanol,CH3CH2CH2OH,has the structure shown below. What is the strongest type of intermolecular force that exists between two propanol molecules? 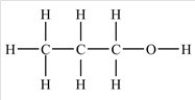
A) London dispersion forces
B) Hydrogen bonding
C) Temporary dipole interactions
D) Dipole-dipole interactions
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q51: STP is defined as a pressure of
Q52: What is the volume of 62.3 g
Q53: Condensation is the opposite of sublimation.
Q54: When octane evaporates,what kind of attractive forces
Q55: The kinetic energy of gas particles _
Q57: Ethylene glycol is expected to be less
Q58: _ are due to momentary changes in
Q59: The stronger the intermolecular forces,the lower the
Q60: What volume does 7.50 × 10<sup>20</sup> molecules
Q61: How many moles are contained in 5.33