Multiple Choice
When octane evaporates,what kind of attractive forces are overcome? 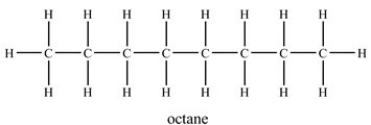
A) Covalent bonding between carbon and hydrogen atoms
B) Hydrogen bonding between octane molecules
C) Dipole-dipole interactions between octane molecules
D) London dispersion forces between octane molecules
Correct Answer:

Verified
Correct Answer:
Verified
Q50: A weather balloon contains 233 L of
Q51: STP is defined as a pressure of
Q52: What is the volume of 62.3 g
Q53: Condensation is the opposite of sublimation.
Q55: The kinetic energy of gas particles _
Q56: Propanol,CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH,has the structure shown below. What is
Q57: Ethylene glycol is expected to be less
Q58: _ are due to momentary changes in
Q59: The stronger the intermolecular forces,the lower the
Q67: Evaporation is an endothermic process.