Multiple Choice
Which statement concerning the reversible reaction 2 NO2(g) 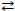 N2O4(g) is true?
N2O4(g) is true?
A) NO2 is the product of the forward reaction.
B) The reverse reaction produces N2O4.
C) At the start of the reaction,the forward and reverse reaction rates are equal.
D) As the forward reaction progresses and more N2O4 is formed,the reverse reaction rate increases.
Correct Answer:

Verified
Correct Answer:
Verified
Q30: When the equilibrium constant for a reversible
Q31: Which value (if any)in each pair corresponds
Q32: Consider the following reversible reaction at equilibrium:
Q33: Which of the following energy quantities is
Q34: A reversible reaction is said to have
Q36: Consider the reaction: C<sub>3</sub>H<sub>8</sub>(g)+ 5 O<sub>2</sub>(g)→ 3
Q37: Which of the following is a true
Q38: Consider the following reversible reaction at equilibrium:
Q39: Which quantity represents the largest amount of
Q40: Increasing the temperature of a reaction mixture