True/False
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 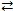 COCl2(g). If chlorine gas is added to the reaction vessel,the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel,the equilibrium will shift to the right.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Which of the following energy quantities is
Q34: A reversible reaction is said to have
Q35: Which statement concerning the reversible reaction 2
Q36: Consider the reaction: C<sub>3</sub>H<sub>8</sub>(g)+ 5 O<sub>2</sub>(g)→ 3
Q37: Which of the following is a true
Q39: Which quantity represents the largest amount of
Q40: Increasing the temperature of a reaction mixture
Q41: Which of the following energy quantities is
Q42: Catalysts accelerate a reaction by _.<br>A)lowering the
Q43: The rate of a chemical reaction increases