Multiple Choice
The Lewis structure shown below is not a valid Lewis structure. What statement best describes the error in the structure? 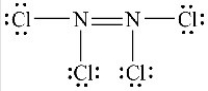
A) The nitrogen atoms violate the octet rule.
B) The chlorine atoms violate the octet rule.
C) The structure contains an incorrect number of valence electrons.
D) Chlorine atoms and nitrogen atoms do not typically form bonds with each other.
Correct Answer:

Verified
Correct Answer:
Verified
Q28: How many covalent bonds are generally formed
Q29: Atoms with three valence electrons generally form
Q30: A N-O bond is more polar than
Q31: The Lewis structure of formaldehyde is shown
Q32: Which of the following elements will generally
Q34: Which is the correct Lewis structure for
Q35: Some covalent compounds are solids,some are liquids,and
Q36: In the valence shell electron pair repulsion
Q37: Which of the following is classified as
Q38: Which molecule's Lewis structure contains an atom