Multiple Choice
The Lewis structure of formaldehyde is shown below. Which statement concerning this structure is INCORRECT? 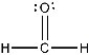
A) Two electrons are being shared between the carbon atom and the oxygen atom.
B) The oxygen atom has four valence electrons that are not being shared with another atom.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have filled valence shells.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: When writing Lewis structures,the symbol below is
Q27: Which statement concerning chemical bonds is FALSE?<br>A)A
Q28: How many covalent bonds are generally formed
Q29: Atoms with three valence electrons generally form
Q30: A N-O bond is more polar than
Q32: Which of the following elements will generally
Q33: The Lewis structure shown below is not
Q34: Which is the correct Lewis structure for
Q35: Some covalent compounds are solids,some are liquids,and
Q36: In the valence shell electron pair repulsion