True/False
The structures shown below are resonance structures of sulfur dioxide. 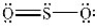 and
and 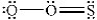
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Phosphorus usually forms two covalent bonds in
Q21: Double bonds and triple bonds are never
Q22: Carbon tetrachloride has _ valence electrons.<br>A)4 (four)<br>B)8
Q23: Nonpolar molecules may contain polar bonds.
Q24: Carbon usually forms four bonds in stable
Q26: When writing Lewis structures,the symbol below is
Q27: Which statement concerning chemical bonds is FALSE?<br>A)A
Q28: How many covalent bonds are generally formed
Q29: Atoms with three valence electrons generally form
Q30: A N-O bond is more polar than