Short Answer
Carbon usually forms four bonds in stable molecules. However unstable carbon compounds with less than four bonds are known. The methyl carbanion shown below is an example. The molecular shape around the carbon atom in this structure is ________. 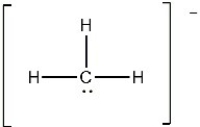
Correct Answer:

Verified
Correct Answer:
Verified
Q19: A molecule that contains only one polar
Q20: Phosphorus usually forms two covalent bonds in
Q21: Double bonds and triple bonds are never
Q22: Carbon tetrachloride has _ valence electrons.<br>A)4 (four)<br>B)8
Q23: Nonpolar molecules may contain polar bonds.
Q25: The structures shown below are resonance structures
Q26: When writing Lewis structures,the symbol below is
Q27: Which statement concerning chemical bonds is FALSE?<br>A)A
Q28: How many covalent bonds are generally formed
Q29: Atoms with three valence electrons generally form