Multiple Choice
Which of the following reactions could be described by the energy diagram below? 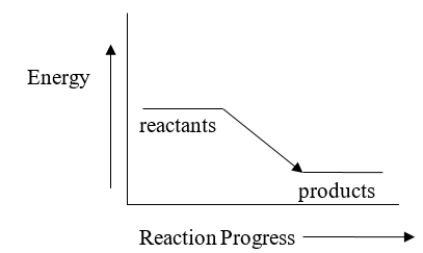
A) 2 CO2 + 556 kJ→ 2 CO + O2
B) CH4 + 2 O2 → CO2 + 2 H2O + heat
C) 8 H2S + heat → 8 H2 + S8
D) 6 CO2 + 6 H2O + heat → C6H12O6 + 6 O2
E) All of the reactions can be described by this energy diagram.
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Which of the following molecules is a
Q43: Which of the following diagrams illustrates an
Q44: How does direct and indirect calorimetry differ?<br>A)
Q45: Combustion reactions are _ because products of
Q46: Select the correct statement regarding the reactants
Q48: Which of the following is a potential
Q49: Which process that occurs during this reaction
Q50: The energy difference between I and II
Q51: The first law of thermodynamics states that<br>A)
Q52: What is a biochemical pathway?<br>A) the path