Multiple Choice
Select the correct statement regarding the reactants and products in the reaction represented by the energy diagram below. 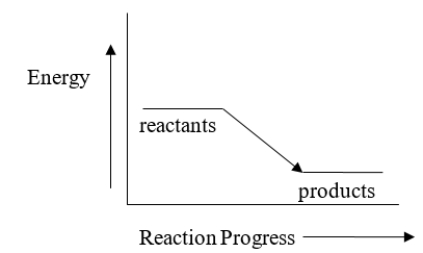
A) The products have more energy than the reactants.
B) The reactants are more stable than the products.
C) The products are more stable than the reactants.
D) The reactants and products have the same energy.
E) The products are less stable than the reactants.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Which statement is the BEST definition of
Q42: Which of the following molecules is a
Q43: Which of the following diagrams illustrates an
Q44: How does direct and indirect calorimetry differ?<br>A)
Q45: Combustion reactions are _ because products of
Q47: Which of the following reactions could be
Q48: Which of the following is a potential
Q49: Which process that occurs during this reaction
Q50: The energy difference between I and II
Q51: The first law of thermodynamics states that<br>A)