Multiple Choice
What is the meaning of the arrow in the following chemical equation? 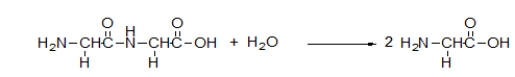
A) The reaction mixture increases in temperature.
B) A product is substituted for a reactant.
C) The reactants collide.
D) The reactants are transformed into the products.
E) The reaction is heated.
Correct Answer:

Verified
Correct Answer:
Verified
Q56: Which of these figures illustrate chemical reactions?
Q57: The following reaction is an example of
Q58: The ionic compound CaCO<sub>3</sub> has a formula
Q59: How many copper atoms are in 0.5
Q60: Which of the following is NOT an
Q62: A common, over-the-counter antacid is Al(OH)<sub>3</sub>.This antacid
Q63: Is this chemical equation balanced? Mg(OH)<sub>2</sub> +
Q64: Cellular respiration is an example of a(n)_
Q65: A molecule of oxygen, O<sub>2</sub>, has a
Q66: If Jane Doe has a blood carbon