Multiple Choice
A common, over-the-counter antacid is Al(OH) 3.This antacid reacts with gastric juice (HCl) in the stomach, producing AlCl3 and H2O.Which of the following equations can be used to correctly determine how much gastric juice (HCl) reacts with an antacid tablet containing 0.25 grams Al(OH) 3? Note that you will need to write a balanced chemical equation to answer this question.
A) 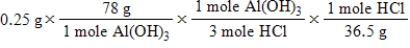
B) 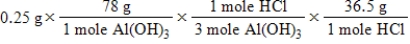
C) 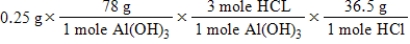
D) 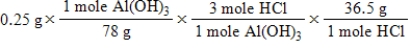
E) 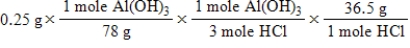
Correct Answer:

Verified
Correct Answer:
Verified
Q57: The following reaction is an example of
Q58: The ionic compound CaCO<sub>3</sub> has a formula
Q59: How many copper atoms are in 0.5
Q60: Which of the following is NOT an
Q61: What is the meaning of the arrow
Q63: Is this chemical equation balanced? Mg(OH)<sub>2</sub> +
Q64: Cellular respiration is an example of a(n)_
Q65: A molecule of oxygen, O<sub>2</sub>, has a
Q66: If Jane Doe has a blood carbon
Q67: What are the products of the reaction