Multiple Choice
What is the molecular geometry of the atoms bonded to oxygen in methanol, shown below? 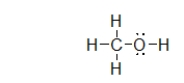
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: How does VSEPR theory explain the electron
Q66: Electrostatic interactions between positive and negative ions
Q67: Which element is the MOST electronegative?<br>A) fluorine<br>B)
Q68: Does propane (shown)or octane (C<sub>8</sub>H<sub>18</sub>)exhibit stronger dispersion
Q69: How many electron groups do the carbon
Q71: What is the angle between groups of
Q72: Does ethanol have a permanent dipole? <img
Q73: _ is the sharing of electrons between
Q74: An atom, X, has a tetrahedral electron
Q75: Which of the following bonds is nonpolar?<br>A)