Multiple Choice
Does propane (shown) or octane (C8H18) exhibit stronger dispersion forces? 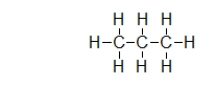
A) Propane.The smaller molecules can get closer to one another.
B) Propane.It has a larger permanent dipole.
C) Octane.It has a larger permanent dipole.
D) Octane.It has more electrons.
E) They both have the same amount of forces.
Correct Answer:

Verified
Correct Answer:
Verified
Q63: Which molecule below exhibits the strongest intermolecular
Q64: What is the H-C-H bond angle of
Q65: How does VSEPR theory explain the electron
Q66: Electrostatic interactions between positive and negative ions
Q67: Which element is the MOST electronegative?<br>A) fluorine<br>B)
Q69: How many electron groups do the carbon
Q70: What is the molecular geometry of the
Q71: What is the angle between groups of
Q72: Does ethanol have a permanent dipole? <img
Q73: _ is the sharing of electrons between