Multiple Choice
Propane is a fuel commonly used in barbeques and to heat homes.It has the structure shown below.Does propane have a permanent dipole? 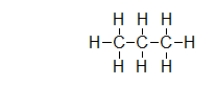
A) Yes.The carbon is partially negative, and the hydrogen is partially positive.
B) Yes.The carbon is partially positive, and the hydrogen is partially negative.
C) Yes.One side of the molecule is always partially positive, and the other side is partially negative.
D) No.Propane has a temporary dipole.
E) No.Propane never displays any dipole at all.
Correct Answer:

Verified
Correct Answer:
Verified
Q18: A polar molecule is one that has<br>A)
Q19: What is the electron geometry of the
Q20: Does chloroform (CHCl<sub>3</sub>)contain polar bonds?<br>A) Yes.All of
Q21: Which figure BEST illustrates how two molecules
Q22: Which BEST describes your reasoning in answering
Q24: How does estradiol stimulate the growth of
Q25: Which of the following covalent compounds is
Q26: Which of the following covalent bonds is
Q27: Which of these molecules exhibits dipole-dipole forces
Q28: How many atoms are bonded to the