Multiple Choice
Which of these molecules exhibits dipole-dipole forces but cannot hydrogen bond with like molecules? 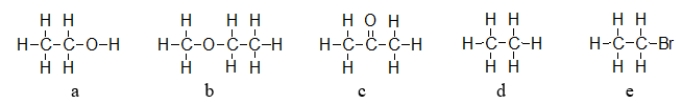
A) b only
B) a, b, and c
C) b, c, and e
D) a, b, c, and e
E) d only
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Which BEST describes your reasoning in answering
Q23: Propane is a fuel commonly used in
Q24: How does estradiol stimulate the growth of
Q25: Which of the following covalent compounds is
Q26: Which of the following covalent bonds is
Q28: How many atoms are bonded to the
Q29: What is the purpose of Valence Shell
Q30: What is the electron geometry of the
Q31: What is the strongest type of intermolecular
Q32: What is the angle between groups of