Multiple Choice
What is the strongest type of intermolecular force of attraction that a caffeine molecule could form with other caffeine molecules? 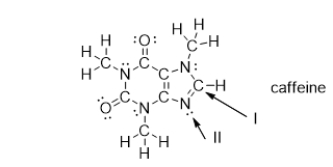
A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions
Correct Answer:

Verified
Correct Answer:
Verified
Q76: Electronegativity is a measure of an atom's
Q77: What is the angle between groups of
Q78: Antiestrogens are one type of molecule that
Q79: What is the molecular geometry of nitrogen
Q80: Which element is the MOST electronegative?<br>A) nitrogen<br>B)
Q81: Why do electron groups around a central
Q82: Is chloroform (CHCl<sub>3</sub>)a polar molecule?<br>A) No.All polarities
Q83: Which of the molecules below exhibits hydrogen
Q84: When determining the shape of a molecule,
Q85: Atom X in a molecule has tetrahedral