Multiple Choice
What is the strongest type of intermolecular interaction attracting molecules of propane together? 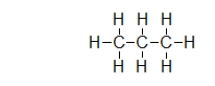
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: Which of the molecules below does NOT
Q50: What is the molecular geometry of the
Q51: An atom in a molecule has one
Q52: Which of the following steps is done
Q53: Ethylene is used as a starting material
Q55: What is the electron geometry of the
Q56: An atom in a molecule has two
Q57: How many nonbonding pairs are on the
Q58: Which of the following interactions is the
Q59: Formaldehyde is used as a preservative and