Multiple Choice
Formaldehyde is used as a preservative and disinfectant.It has the structure shown below.What is the strongest type of intermolecular interaction attracting molecules of formaldehyde together? 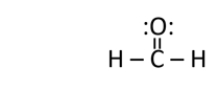
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
Correct Answer:

Verified
Correct Answer:
Verified
Q54: What is the strongest type of intermolecular
Q55: What is the electron geometry of the
Q56: An atom in a molecule has two
Q57: How many nonbonding pairs are on the
Q58: Which of the following interactions is the
Q60: An atom in a molecule has a
Q61: Which figure BEST illustrates how two molecules
Q62: What is the electron geometry of the
Q63: Which molecule below exhibits the strongest intermolecular
Q64: What is the H-C-H bond angle of