Multiple Choice
Which molecules exhibit the strongest intermolecular forces of attraction? 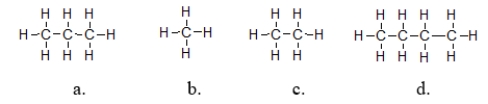
A) structure a
B) structure b
C) structure c
D) structure d
E) They all have the same strength of intermolecular force because they are all nonpolar molecules
Correct Answer:

Verified
Correct Answer:
Verified
Q8: What is the molecular geometry of each
Q9: How is estradiol recognized by the estrogen
Q10: Which elements have the highest electronegativity and
Q11: What is the molecular geometry of carbon
Q12: The structure below is a Lewis structure
Q14: Tamoxifen has some key similarities to estradiol.Which
Q15: The electronegativity difference between C and H
Q16: The only interactions between two or more
Q17: Chloroform (CHCl<sub>3</sub>)is an anesthetic and is also
Q18: A polar molecule is one that has<br>A)