Multiple Choice
The structure below is a Lewis structure of methane.This Lewis structure tells us many things about methane that are useful.However, it also suggests one characteristic of methane that is, in fact, false.What is this one characteristic? 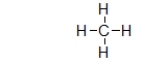
A) The Lewis structure suggests that carbon has a full valence shell, but it really doesn't.
B) The Lewis structure suggests that carbon has four bonds, but it really doesn't.
C) The Lewis structure suggests that carbon is bonded to four hydrogens, but it really isn't.
D) The Lewis structure suggests that methane is flat, but it really isn't.
E) The Lewis structure suggests that methane is stable, but it really isn't.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The electronegativity difference between C and O
Q8: What is the molecular geometry of each
Q9: How is estradiol recognized by the estrogen
Q10: Which elements have the highest electronegativity and
Q11: What is the molecular geometry of carbon
Q13: Which molecules exhibit the strongest intermolecular forces
Q14: Tamoxifen has some key similarities to estradiol.Which
Q15: The electronegativity difference between C and H
Q16: The only interactions between two or more
Q17: Chloroform (CHCl<sub>3</sub>)is an anesthetic and is also