Multiple Choice
What is the electron geometry of the carbon in formaldehyde, shown below? 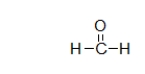
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: How many nonbonding pairs are on the
Q58: Which of the following interactions is the
Q59: Formaldehyde is used as a preservative and
Q60: An atom in a molecule has a
Q61: Which figure BEST illustrates how two molecules
Q63: Which molecule below exhibits the strongest intermolecular
Q64: What is the H-C-H bond angle of
Q65: How does VSEPR theory explain the electron
Q66: Electrostatic interactions between positive and negative ions
Q67: Which element is the MOST electronegative?<br>A) fluorine<br>B)