Multiple Choice
Which of the bonds in the following molecule are polar covalent? 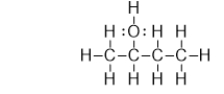
A) C-H and O-H only
B) C-O and O-H only
C) C-O only
D) C-C and C-O
E) O-H only
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: How many atoms are bonded to the
Q29: What is the purpose of Valence Shell
Q30: What is the electron geometry of the
Q31: What is the strongest type of intermolecular
Q32: What is the angle between groups of
Q34: In addition to single bonds, which of
Q35: The electronegativity difference between K and Cl
Q36: Which bonds in caffeine are polar covalent
Q37: Which bond is correctly labeled with a
Q38: What is the electron geometry of the