Multiple Choice
What is the electron geometry of the electron groups on the oxygen atom in methanol, shown below? 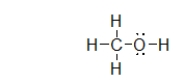
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Which of the bonds in the following
Q34: In addition to single bonds, which of
Q35: The electronegativity difference between K and Cl
Q36: Which bonds in caffeine are polar covalent
Q37: Which bond is correctly labeled with a
Q39: What is the molecular geometry of the
Q40: What is the molecular geometry of the
Q41: What is the molecular geometry of the
Q42: Which type(s)of molecular model illustrate the three-dimensional
Q43: Which element is the LEAST electronegative?<br>A) potassium<br>B)