Multiple Choice
In the energy level diagram shown in the figure below, the electron is excited to the E2 energy level.If the atom absorbs a photon with the exact wavelength to move the electron to another energy level, which energy level would correspond to the incoming photon with the largest wavelength? 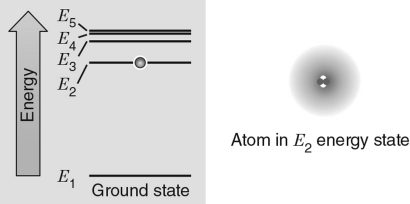
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: A certain light wave has a frequency
Q6: As wavelength increases, the energy of a
Q7: If the frequency of a beam of
Q8: Astronomers have now found a large number
Q9: A red photon has a wavelength of
Q11: The difference in energy between the n
Q12: Why do we see black lines in
Q13: Which of these planets would be expected
Q14: If you are standing in a fixed
Q15: In the energy level diagram shown in