Essay
The difference in energy between the n = 2 and n =1 electronic energy levels in the hydrogen atom is 1.6 *10-18 J.If an electron moves from the n = 1 level to the n=2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown in the figure below to answer this question. 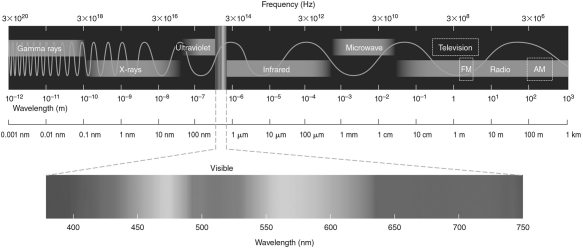
Correct Answer:

Verified
The n =2 energy level is higher than the...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: As wavelength increases, the energy of a
Q7: If the frequency of a beam of
Q8: Astronomers have now found a large number
Q9: A red photon has a wavelength of
Q10: In the energy level diagram shown in
Q12: Why do we see black lines in
Q13: Which of these planets would be expected
Q14: If you are standing in a fixed
Q15: In the energy level diagram shown in
Q16: Describe, in your own words, why electrons