Multiple Choice
A compression, at a constant pressure of 190 kPa, is performed on 5.0 moles of an ideal monatomic gas. The compression reduces the volume of the gas from 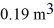 to
to 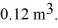 The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
A) -13 kJ.
B) 13 kJ.
C) -33 kJ.
D) 33 kJ.
E) 0.00 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: How many grams of ice at -13°C
Q3: A heat conducting rod, 0.90 m long,
Q13: A person pours 330 g of water
Q14: During an isothermal process,5.0 J of heat
Q24: What is the net power that a
Q34: A substance has a melting point of
Q38: A copper cylinder with a mass of
Q44: An ideal monatomic gas cools from 455.0
Q52: A cylinder contains 23 moles of an
Q55: A person makes ice tea by adding