Short Answer
If a certain sample of an ideal gas has a temperature of 109°C and exerts a pressure of 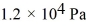 on the walls of its container, how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is
on the walls of its container, how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is
6.022 × 1023 molecules/mol.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: 1)000 L of water at 20.00°C will
Q11: Which contains more moles of material: 80
Q16: A bag of potato chips contains 2.00
Q17: A 3.2-L volume of neon gas (Ne)is
Q20: The figure shows a pV diagram for
Q24: A brass rod is 40.1 cm long
Q28: The number of molecules in one mole
Q37: A sealed container holds 0.020 moles of
Q43: 3.00 moles of an ideal gas at
Q53: When a solid melts,<br>A) the temperature of