Multiple Choice
A sealed 89- 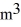 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Suppose that a steel bridge,1000 m long,was
Q10: (a)Internal human body temperature is often stated
Q27: 2.0 L of an ideal nitrogen gas
Q30: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg" alt="A sealed
Q31: The figure shows a pV diagram for
Q32: Two identical concrete slabs lie flat and
Q33: What is the mass density of argon
Q35: The figure shows a pV diagram for
Q37: The figure (not to scale) shows a
Q41: A certain metal has a coefficient of