Multiple Choice
The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 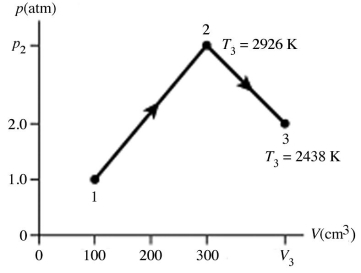
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Suppose that a steel bridge,1000 m long,was
Q8: How many moles of water (H<sub>2</sub>O)molecules are
Q27: 2.0 L of an ideal nitrogen gas
Q27: A vertical tube that is closed at
Q30: A sealed 26- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg" alt="A sealed
Q32: Two identical concrete slabs lie flat and
Q33: What is the mass density of argon
Q34: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg" alt="A sealed
Q35: The figure shows a pV diagram for
Q46: An ideal gas is at a pressure