Multiple Choice
Scenario
While radioactive isotopes are used in medicine to identify tumors and other diseases, they can also be used to treat diseases such as cancer. One method to treat cancerous tumors is to expose them to radiation which can kill the cancerous cells and the tumor. In 2013, the U.S. Food and Drug Administration approved a new cancer treatment based on the radioactive isotope radium-223; this isotope has a half-life of 11.4 days. The radioactive drug, known as Xofigo®, is injected into the patient's bloodstream and travels to certain regions of the body. Because it emits high-energy radiation over short distances, it can kill cancerous cells in the sites where it localizes.
After the drug was injected into a person, the following data were collected regarding the amount of radiation measured in different organs in the person's body.
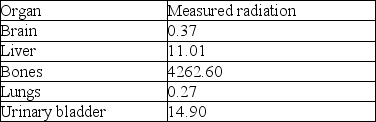
-Using the above table, what kind of cancer do you think Xofigo® is used to treat?
A) liver cancer
B) bone cancer
C) brain cancer
D) lung cancer
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Isotopes of an element have the same
Q29: Adjacent water molecules interact through the _.<br>A)sharing
Q30: Which of the following elements is a
Q31: What are the reactant(s)in the following chemical
Q32: _ is an example of an element.<br>A)Water<br>B)Nitrogen<br>C)Glucose<br>D)Salt
Q34: What is the outcome of evaporative cooling
Q35: The lower the pH of a solution,
Q36: An uncharged atom of mercury has an
Q37: Why is water considered a polar molecule?<br>A)The
Q38: The hydrogen and oxygen atoms of a