Multiple Choice
The figure shows a pV diagram for 0.98 mol of ideal gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? (R = 8.31 J/mol ∙ K) . 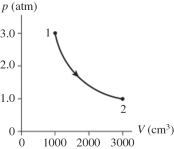
A) -160°C
B) 12°C
C) 380°C
D) 110°C
Correct Answer:

Verified
Correct Answer:
Verified
Q16: A refrigerator has an interior volume of
Q30: A large vat contains 1.000 L of
Q33: A camper is about to drink his
Q47: An ideal gas occupies 6.00 × 10<sup>2</sup>
Q51: The coefficient of linear expansion of copper
Q65: For the mercury in a thermometer to
Q66: A gas-filled vertical cylinder,closed at the bottom
Q90: A mole of diatomic oxygen molecules and
Q94: On his honeymoon,James Joule attempted to explore
Q189: A substance has a melting point