Multiple Choice
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the change of internal energy over the full cycle 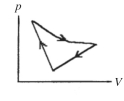
A) is positive.
B) is negative.
C) is zero.
D) cannot be determined from the information given.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: A compression, at a constant pressure
Q31: An ideal Carnot heat engine operates
Q32: The figure shows a pV diagram for
Q33: An ideal Carnot engine is operated
Q35: An ideal Carnot heat engine has
Q45: If the efficiency of a reversible engine
Q58: An air conditioner with a coefficient of
Q71: Which of the following is a false
Q79: In an isochoric process,the internal (thermal)energy of
Q93: At what,if any,temperature are the numerical readings