Multiple Choice
Use the following figure to answer the question.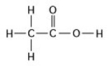
Two moles of the compound in the figure would weigh how many grams? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
A) 30
B) 60
C) 90
D) 120
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What is the hydroxyl ion (OH⁻) concentration
Q3: How much of 0.5 M glucose (molecular
Q4: Cohesion, surface tension, and adhesion are the
Q5: Melting of ice and thus reduced feeding
Q6: Why does ice float in liquid water?<br>A)
Q7: Which of the following is considered to
Q8: As the [H₃O⁺] of the solution decreases,
Q9: Water molecules can form hydrogen bonds with
Q10: One of the buffers that contribute to
Q11: A 0.01 M solution of a substance