Multiple Choice
Suppose that a gas satisfies the universal gas law, 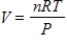 with n equal to 40 moles of the gas and R, the universal gas constant, equal to 0.082054. What is V if
with n equal to 40 moles of the gas and R, the universal gas constant, equal to 0.082054. What is V if 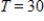 (kelvins, the units in which temperature is measured on the Kelvin scale) and
(kelvins, the units in which temperature is measured on the Kelvin scale) and 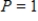 atmosphere? Round your answer to four decimal places.
atmosphere? Round your answer to four decimal places.
A) 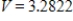
B) 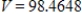
C) 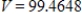
D) 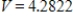
E) 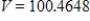
Correct Answer:

Verified
Correct Answer:
Verified
Q109: Suppose that the joint cost function in
Q110: Suppose that the following table shows the
Q111: Suppose wind and cold temperatures combine to
Q112: Test <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1243/.jpg" alt="Test for
Q113: Test <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1243/.jpg" alt="Test for
Q114: Find the values for each of the
Q115: In economics, the most economical quantity Q
Q116: If <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1243/.jpg" alt="If ,
Q117: Test for relative maximum and minimum.
Q118: Suppose that a manufacturer produces two brands