Multiple Choice
In the diagram below, one beaker contains pure water and the other contains an equal volume of seawater. Seawater has various salts dissolved in it. The beakers are sitting in a totally enclosed chamber, and the outside temperature and pressure are held constant. Identify the statements below about this situation that are not correct. 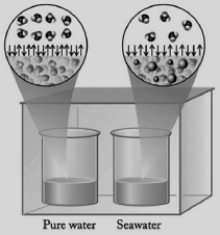
I. Water will be transferred from the pure water beaker to the seawater beaker.
II. Water will be transferred from the seawater beaker to the pure water beaker.
III. The vapor pressure of the pure water is higher than the vapor pressure of the seawater.
IV. Pure water evaporates at a faster rate than seawater.
V. Water in the gas phase condenses into both beakers at the same rate.
A) II only
B) II and V
C) II, IV, and V
D) II, III, and IV
E) I, III, and IV
Correct Answer:

Verified
Correct Answer:
Verified
Q8: How many moles of solute are there
Q16: Considering that ideal behavior is not always
Q93: Identify the following statement as true or
Q102: A 376 mg sample of a nonelectrolyte
Q103: The pressure inside a bottle of carbonated
Q106: Which of the following is a colligative
Q107: Which solution will have the lowest osmotic
Q110: The arrow in the diagram below indicates
Q112: Which graph best describes how the vapor
Q135: Which one of the ionic compounds below