Multiple Choice
Of the two compounds shown below, one is slightly soluble in water and the other is insoluble in water. Identify the slightly soluble compound and explain why it is soluble in water.
I. ClCH2CH2Cl 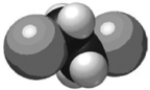
II. CHCl2CH3 
A) I because it can form hydrogen bonds with water at each end of the molecule.
B) I because it has stronger dispersion interactions with water.
C) II because it has stronger dipole-dipole interactions with water.
D) II because it has stronger dispersion interactions with water.
E) I because it is nonpolar.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Which of the following compounds will have
Q52: Which of the following gases would you
Q74: The boiling point of HBr is higher
Q76: Define the term vapor pressure.
Q78: The phase diagram for carbon dioxide is
Q82: Which hydride do you predict has the
Q83: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q84: Which of the following substances has a
Q125: Which of the following statements does not
Q151: Which is the dominant interaction between oxygen