Multiple Choice
The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature (22oC) and 100 atm pressure? 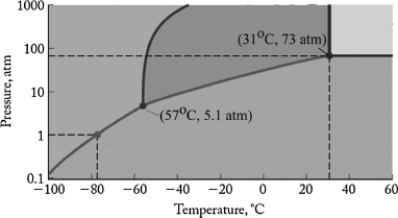
A) gas
B) liquid
C) solid I
D) supercritical fluid
E) solid II
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Which of the following compounds will have
Q41: Which alkane compound has the highest boiling
Q52: Which of the following gases would you
Q74: The boiling point of HBr is higher
Q76: Define the term vapor pressure.
Q79: Of the two compounds shown below, one
Q82: Which hydride do you predict has the
Q83: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q125: Which of the following statements does not
Q151: Which is the dominant interaction between oxygen