Multiple Choice
Point d in the phase diagram below is the ________ 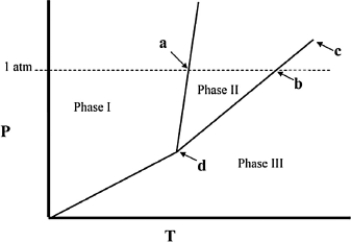
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Which of the following substances would you
Q10: Arrange the following compounds in order of
Q17: Water forms a concave meniscus in a
Q40: Arrange the first four halogens in order
Q71: For molecules or atoms with the same
Q99: The aroma from almonds and cherries is
Q105: The relative energies (strengths) of the intermolecular
Q106: Consider the phase diagram for a substance
Q121: For a molecule to exhibit dipole-dipole interactions,
Q164: Explain why the rate of evaporation of