Multiple Choice
Consider the phase diagram for a substance such as the one shown here. If the line between the solid and liquid phases has a negative slope, then the solid phase is ________ than the liquid phase. 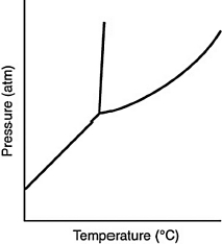
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Arrange the following compounds in order of
Q17: Water forms a concave meniscus in a
Q40: Arrange the first four halogens in order
Q44: Which molecule does not exhibit hydrogen bonding?<br>A)HF<br>B)CH<sub>3</sub>NH<sub>2</sub><br>C)CH<sub>3</sub>OH<br>D)(CH<sub>3</sub>)<sub>3</sub>N<br>E)CH<sub>3</sub>CH<sub>2</sub>OH
Q103: Point d in the phase diagram below
Q105: The relative energies (strengths) of the intermolecular
Q109: In understanding why group 16 hydrides, other
Q110: Given the van der Waals a constant
Q121: For a molecule to exhibit dipole-dipole interactions,
Q164: Explain why the rate of evaporation of