Multiple Choice
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as carbon dioxide is heated from -90oC to 50oC, at 1 atm pressure? 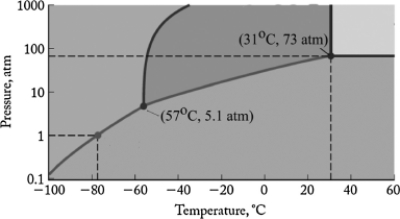
A) solid →Gas
B) solid →Liquid →Gas
C) liquid →Gas
D) gas →Liquid →Solid
E) gas →Liquid
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Would water rise to the same height
Q71: For molecules or atoms with the same
Q88: Arrange the three ionic compounds sodium chloride,
Q89: The relative energies (strengths) of the intermolecular
Q94: Ion interaction energies are determined by
Q95: Sublimation occurs in going from region _
Q115: Of all the noble gases, _ has
Q123: What structural characteristics must a molecule have
Q153: Which is the dominant interaction that leads
Q160: Indicate which of the following pairs of