Multiple Choice
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as the pressure is increased starting at 0.5 atm, and the temperature is kept at -80oC? 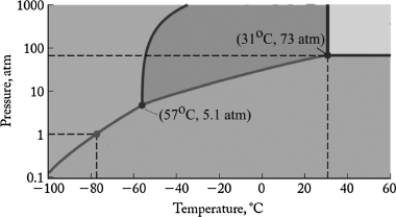
A) solid
Gas
B) solid
Liquid
Gas
C) liquid
Gas
D) gas
Solid
E) gas
Liquid
Correct Answer:

Verified
Correct Answer:
Verified
Q48: Which alcohol should be most soluble in
Q48: Condensation occurs in going from region _
Q52: Which of the following representations best shows
Q55: Rank the following compounds in order of
Q56: The relative energies (strengths) of the intermolecular
Q57: Normal boiling points of branched alkane hydrocarbons
Q58: The relative energies (strengths) of the intermolecular
Q74: Which of the following will require the
Q94: The vapor pressure of a liquid increases
Q154: Which of the following compounds would you