Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the lowest melting point? 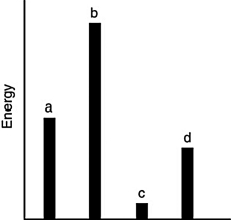
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: Which liquid, water or ethanol, would you
Q53: The phase diagram for carbon dioxide
Q53: Based on their boiling points, which of
Q55: Rank the following compounds in order of
Q56: The relative energies (strengths) of the intermolecular
Q57: Normal boiling points of branched alkane hydrocarbons
Q60: The temperature at point b in the
Q60: Which of the following requires the smallest
Q80: Identify the dominant intermolecular interaction(s) that must
Q154: Which of the following compounds would you