Multiple Choice
Which of these molecules have a dipole moment? 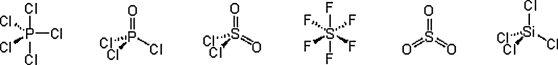
A) PCl5 and SiCl4
B) POCl3, SO2Cl2, and SO3
C) POCl3 and SO2Cl2
D) PCl5, POCl3, SOCl2, SO3, and SiCl4
E) PCl5, SF6, SO3, and SiCl4
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Benzene (C<sub>6</sub>H<sub>6</sub>) is a cyclic, nonpolar molecule.
Q16: Which statement regarding a pi bond between
Q17: Which of these molecules is chiral? <img
Q20: Identify the hybridization of atomic orbitals for
Q21: Which statement A-D about VSEPR theory is
Q23: Which of the following has a central
Q71: Both cyclohexane (C<sub>6</sub>H<sub>12</sub>) and benzene (C<sub>6</sub>H<sub>6</sub>) have
Q93: Which of the following molecules or ions
Q127: Both diazene (N<sub>2</sub>H<sub>2</sub>) and hydrazine (N<sub>2</sub>H<sub>4</sub>) have
Q182: Draw a structure showing the geometry of