Essay
Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below. Explain why you think this molecule is planar or nonplanar. 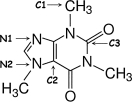
Correct Answer:

Verified
N1: sp2; N2: sp3; C1: sp3; C2 and...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q16: Which statement regarding a pi bond between
Q17: Which of these molecules is chiral? <img
Q18: Which of these molecules have a dipole
Q21: Which statement A-D about VSEPR theory is
Q23: Which of the following has a central
Q25: Broccoli, cabbage, and kale contain compounds that
Q71: Both cyclohexane (C<sub>6</sub>H<sub>12</sub>) and benzene (C<sub>6</sub>H<sub>6</sub>) have
Q93: Which of the following molecules or ions
Q134: Which of the following molecules has a
Q182: Draw a structure showing the geometry of