Multiple Choice
Oxygen has two common molecular anions: peroxide (O22-) and superoxide (O2-) . Use the MO energy level diagram below to identify which one of the following statements is not correct. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 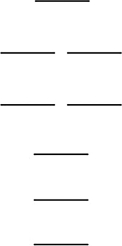
A) The bond order of the peroxide is 1.
B) The superoxide has a shorter bond than the peroxide.
C) Like O2, the peroxide is paramagnetic.
D) The superoxide has a stronger bond than the peroxide.
E) Oxygen, O2, has a stronger bond than either of these oxides.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The amide group is an important structural
Q22: Which of the following compounds is the
Q30: What is the hybridization of the bromine
Q58: Which of the following is a planar
Q63: Identify the hybridization of the atomic orbitals
Q64: Which of the following diagrams shows the
Q65: Use energy levels of diatomic molecules derived
Q66: The tetrahedral bond angle is _.<br>A) 90<sup>o</sup><br>B)
Q72: Which of the following shows the orientation
Q119: Predict the bond order of the NO<sup>+</sup>