Short Answer
Identify the hybridization of the atomic orbitals on carbon atoms labeled 1, 2, and 3. The hydrogen atoms and lone-pair electrons are not shown in the diagram. 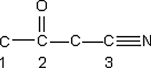
Correct Answer:

Verified
C1: sp3; C2...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: Which type of molecular orbital has maximum
Q11: What hybridization is needed to describe the
Q27: Which one of the statements A-D about
Q58: Which of the following is a planar
Q61: The amide structure is the fundamental linking
Q62: What is the geometry of the ClF<sub>4</sub><sup>-</sup>
Q64: Which of the following diagrams shows the
Q65: Use energy levels of diatomic molecules derived
Q66: The tetrahedral bond angle is _.<br>A) 90<sup>o</sup><br>B)
Q68: Oxygen has two common molecular anions: peroxide